Wednesday, 8 April 2020
Spectacular advances in two-photon microscopy and two-photon calcium imaging
What if I told you that scientists had just succeeded in recording the simultaneous activity of 12 000 neurons in the cortex of a mouse as it moved freely around its cage, and that they had done so at the cellular level, down to a frequency of 17 Hertz? Would you say something like, “So what?” or “Who cares?” or “Why don’t you tell me in language I can understand?” In this post, I’m going to try to meet that last challenge.
In the mid-20th century, scientists discovered that using very small glass or metal electrodes, they could record the electrical activity of neurons, either by inserting the electrodes into them, or by mounting electrodes externally close to small groups of neurons. Both of these methods entail problems and technical challenges. For example, inserting an electrode into a cell to record its activity can damage many things, including the cell itself. Electrodes mounted externally can record the activity of only a small number of cells at a time and can’t capture very precise information about the source of the activity.
In the early 1980s researchers began developing another approach, calcium imaging, by which the activity of nerve cells could be pictured directly. Calcium is an ion (Ca+2) that flows naturally into a neuron when it is excited by other neurons. The influx of calcium ions then makes this neuron produce nerve impulses. The details, of course, are somewhat more complicated. Calcium can also be released from all kinds of compartments inside the cell itself. But in general, the concentration of calcium at certain points inside a neuron can be multiplied by 10 or even 100 when it receives excitatory signals from other neurons. This process also enables the mechanisms of synaptic plasticity to come into play.
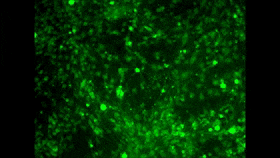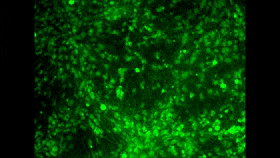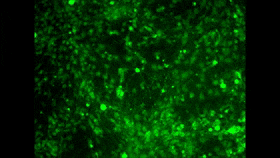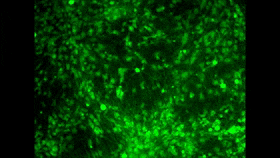
In the 1980s and 1990s, researchers began developing two tools that ultimately led to the achievement that I mentioned at the start of this post. The first of these tools consisted of fluorescent molecules whose fluorescence changes with the concentration of calcium in their environment. I won’t take time here to describe the various kinds of molecules that can exhibit this property, but I will mention that one of the problems in using them as a tool is to find a way to introduce them into large populations of cells simultaneously. And maybe you see where I’m headed here: one of the great strengths of calcium imaging is that it does let you see the activity of countless neurons at the same time (for example, in the image above, which doesn’t come from the study I’m discussing here). And as you may have surmised by now, researchers have in fact succeeded in passing fluorescent calcium molecules through cell membranes and even in manipulating cells genetically so that they manufacture such molecules themselves.
Microscopy was the other field in which some major technological developments were needed for scientists to be able to view, in real time, the fluctuations in calcium ions that correspond to neural activity. These developments included confocal microscopy and charge-coupled device (CCD) video cameras, but one of the most powerful has been two-photon microscopy. Ordinary confocal microscopy uses a single one-photon laser beam to excite fluorescent calcium molecules. Two-photon microscopy uses two such beams. Each of them has a lower energy level than is needed to activate the fluorescent molecules. But at specific locations, the two beams interfere with each other in such a way that their energies are summed, thus exciting the molecules enough for them to emit light.
Two-photon microscopy has many advantages, one of which is that the laser beams cause less damage to the cells. But most importantly, this method makes it possible to record fluorescence, and hence neural activity, to a depth of several hundred microns below the surface of the cortex. Researchers have even invented a miniature helmet that a mouse can wear as its moves around its cage, while a miniature two-photon microscope inside the helmet records the flows of calcium through even the tiniest structures in the mouse’s cortical neurons.
But back to the study that I mentioned at the start. It was published in the journal Cell in April 2019, and its title is ”Volumetric Ca2+ Imaging in the Mouse Brain Using Hybrid Multiplexed Sculpted Light Microscopy”. And as I said, the researchers in this study successfully recorded the activity of nearly 12 000 neurons at the same time. They did so within 1 mm x 1 mm x 1.22 mm spaces at various locations in a mouse’s cortex while it calmly went about its daily life. If you don’t think that’s amazing, then I don’t think anything will impress you!
From the Simple to the Complex | Comments Closed








